In general, improvers and mixes consist of foodstuffs, such as grain products, milk products, sugar and types of sugar, fats and oilseeds, for example (cf. Backmittelinstitut e.V. 1999).
Moreover, improvers contain additives which, due to their technological effect, contribute decisively to simplifying and enhancing the production of baked goods. These are substances which have an effect on baking and which are only used in very small quantities such as, for example, emulsifiers (lecithins, mono- and diacetyl tartaric acid esters of mono- and diglycerides of fatty acids), thickeners and gelling agents (pectin, guar gum) and minerals, together with flour treatment agents (ascorbic acid).
Classification
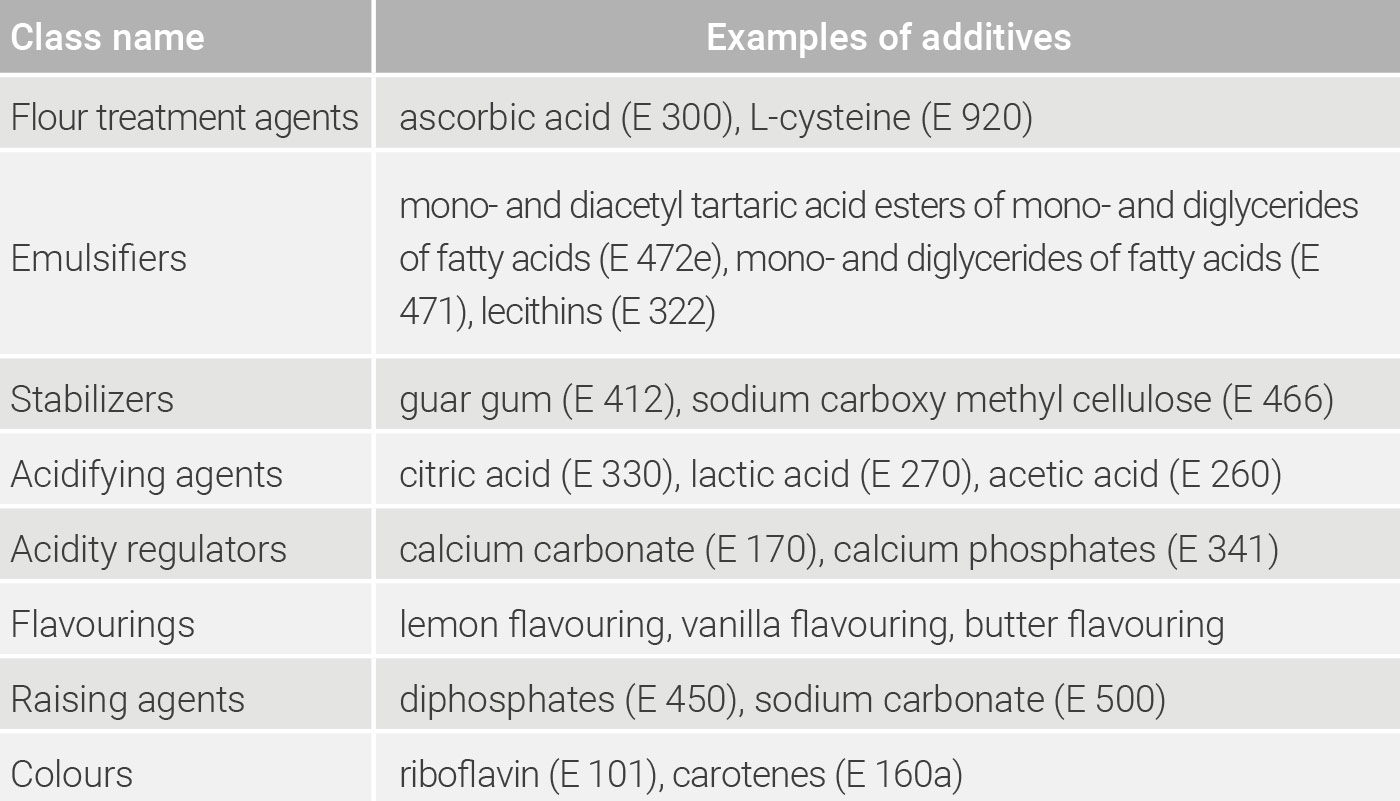
In addition to the number, additives are divided into groups of active ingredients (classes). There are 27 classes of additives altogether, each of which reflects the main function. An overview of the different classes with examples of additives used is given in table 3.2.
Meaning of the E number
The European Union allocates a corresponding number after the approval of additives. Here the E stands for Europe. The E number is a code and serves, irrespective of the language of a country, to unambiguously categorize the relevant substance. The order of the number is of no significance. Substances with the partially listed letters of a – h belong to the same family of substances, but have been approved independently.
Approval prerequisites for every additive: The most stringent tests and trial procedures for the health risk assessment
In the European Union, agreed purity criteria apply which guarantee that every additive is checked in great detail assessing its risk to health.
All additives used in the EU have to be safe. It is only after several examinations regarding their risk to health, extensive toxicological tests and the consideration of various safety factors that no negative effects on health are present (no effect level), can an approval come into question. The approval procedure is regulated within an EU regulation. In Germany, the assessment of the risk to health is carried out by the “Bundesinstitut für Risikobewertung (BfR)” (German Federal Institute for Risk Assessment) and in Europe by the European Food Safety Authority (EFSA). In this case, a large safety factor is once again used, namely only 1/100 of the no effect level as a maximum dose for a human being. This is the ADI value (acceptable daily intake).