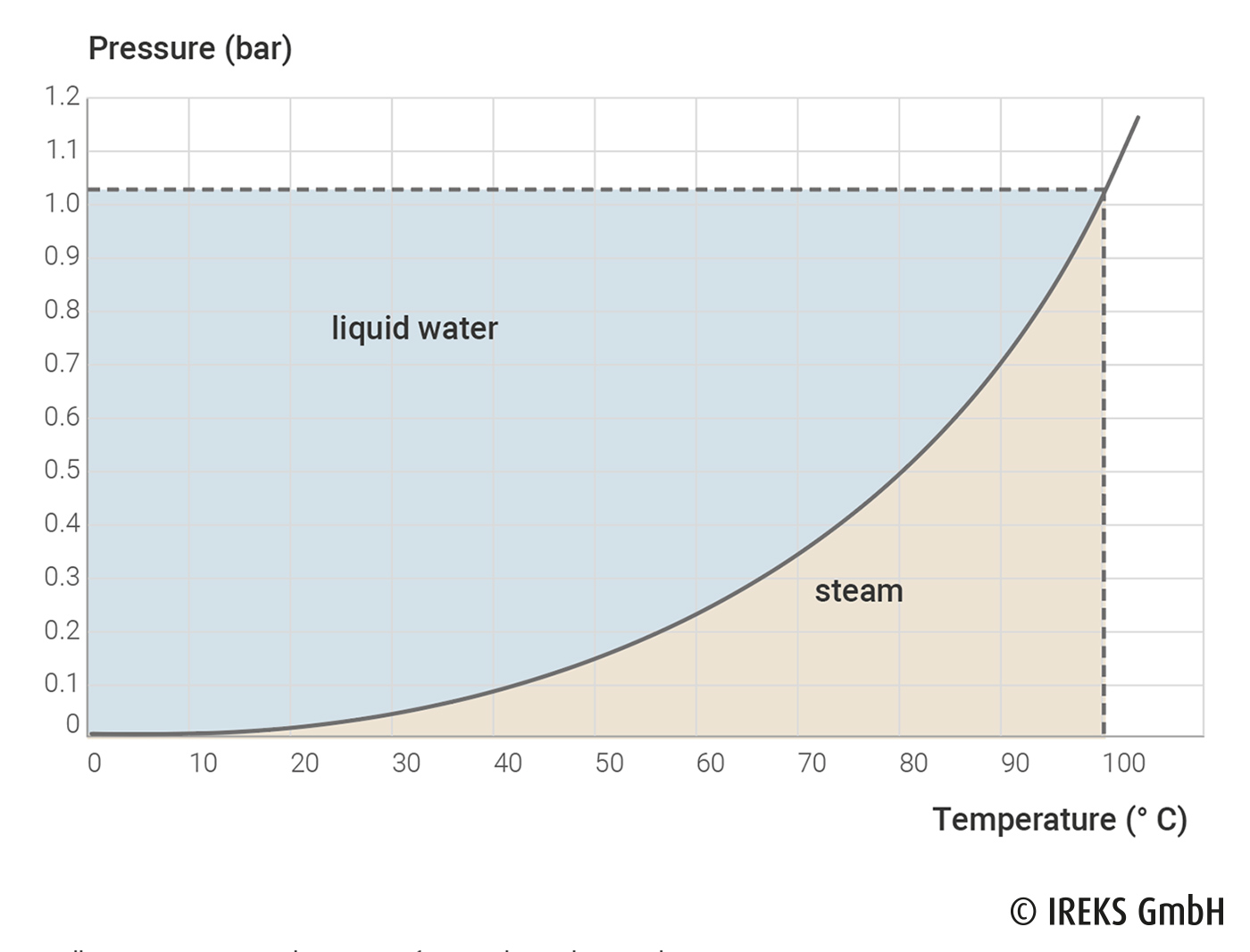
In vacuum conditioning, the fact that the boiling temperature of water depends on the pressure is used. This dependency is shown in illustration 14.1.
In the case of vacuum conditioning, pressures of 30 – 260 mbar are used depending on the installations. With a pressure of 30 mbar, the boiling point of water is around 25° C, with a pressure of 260 mbar approx. 63° C. The pressure created as well as the duration of the cooling process in the vacuum chamber depend on the size and texture of the baked goods together with the required product properties.
Due to the reduction of pressure, a part of the water contained in the product evaporates and, in this way, removes heat from the product, as energy is required for the change of the aggregate state from liquid to gas. This energy is also called evaporation enthalpy.
Here, the product cools from the inside to the outside. This means that, depending on the product, a core temperature of 35 – 55° C is reached within two to six minutes, according to the installation. After cooling, the pressure balance can take place using sterile, cooled and filtered air, whereby a very good microbial stability of the baked goods can be achieved. As a result of this treatment, the products are suitable for packaging in foil bags and storage at room temperature for several days.
In the case of some installations, the possibility also exists to carry out re-moistening of the products via the setting of the air humidity when balancing pressure (cf. König Maschinen Gesellschaft m.b.H. 2020; Schünemann et al. 2016; Werner & Pfleiderer Lebensmitteltechnik GmbH 2012).