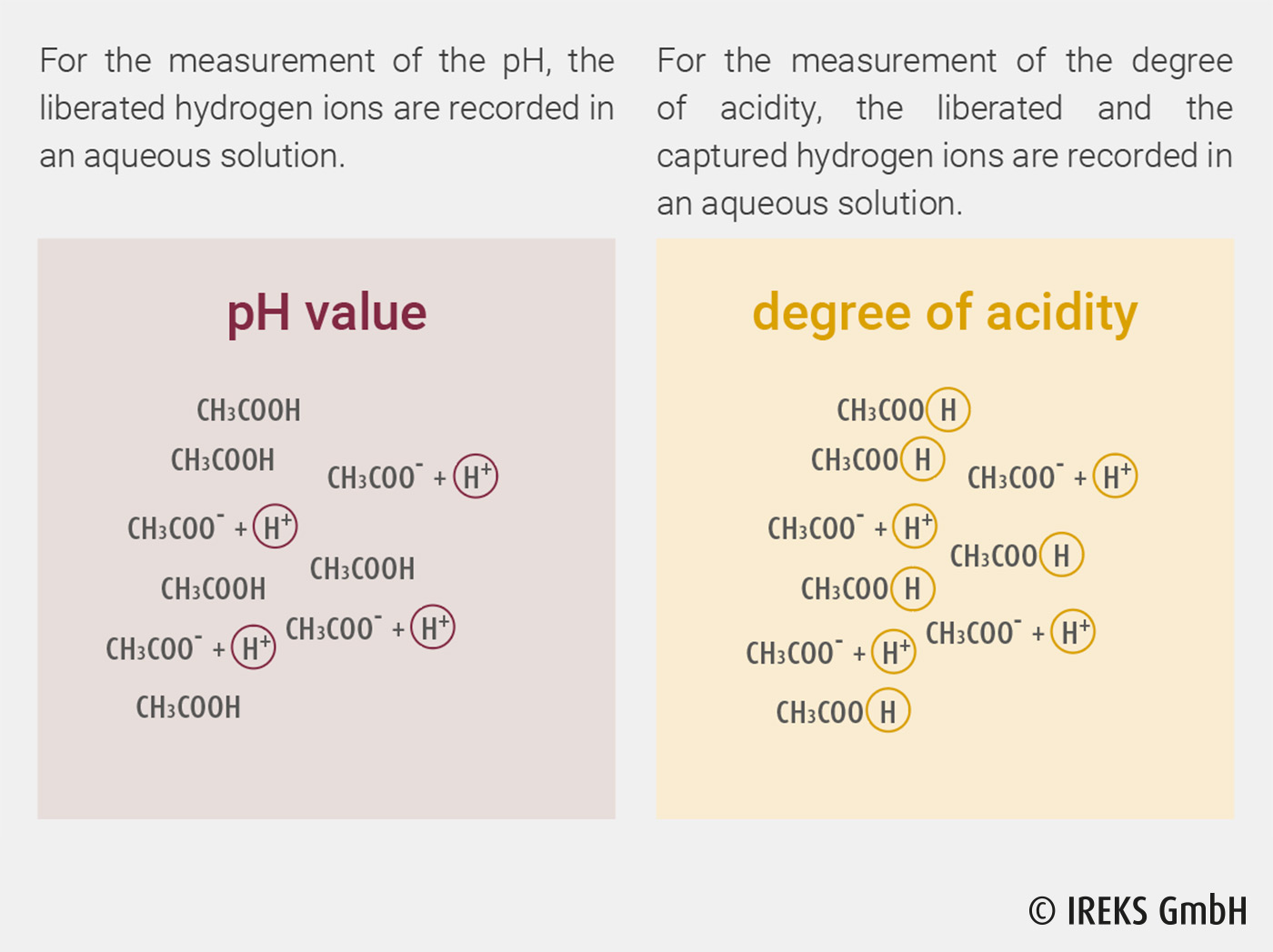
Two measurement variables are used to characterize sourdoughs in foodstuffs, the pH value and the degree of acidity, which are normally determined in aqueous solutions. The pH value is a measure of the strength of an acid, it describes the dissociated (liberated) hydrogen ion (H+). On the other hand, the degree of acidity gives the quantity of an acid and describes both the dissociated and the undissociated (captured) hydrogen ion. In illustration 8.1, it is shown as an example which hydrogen ions of acetic acid (CH3COOH) are recorded for the determination of the pH value and the degree of acidity.
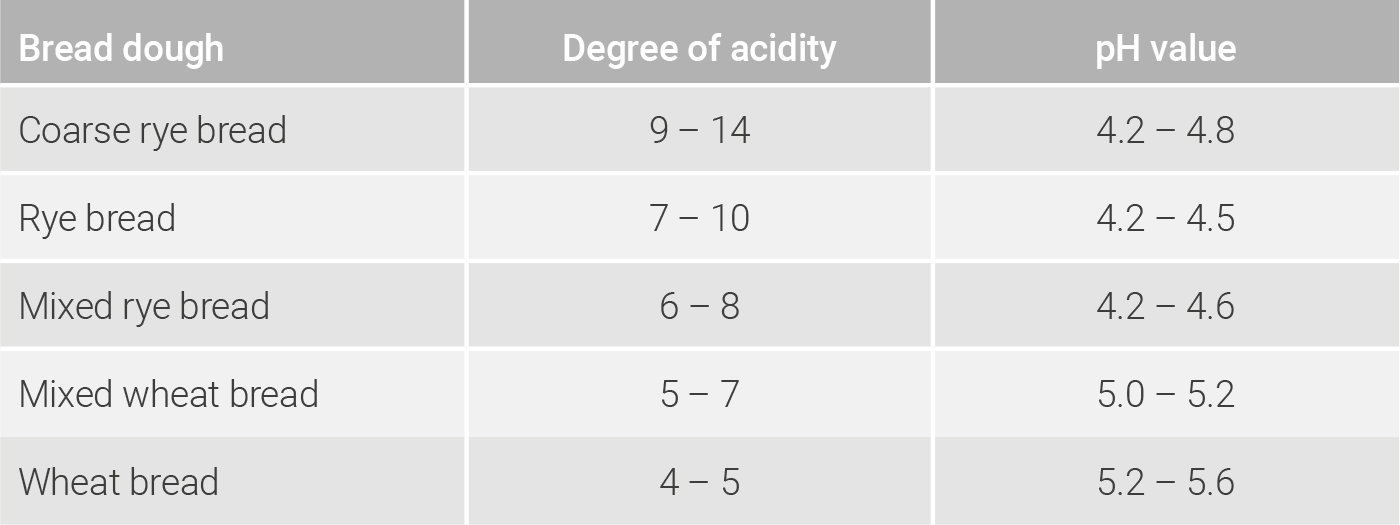
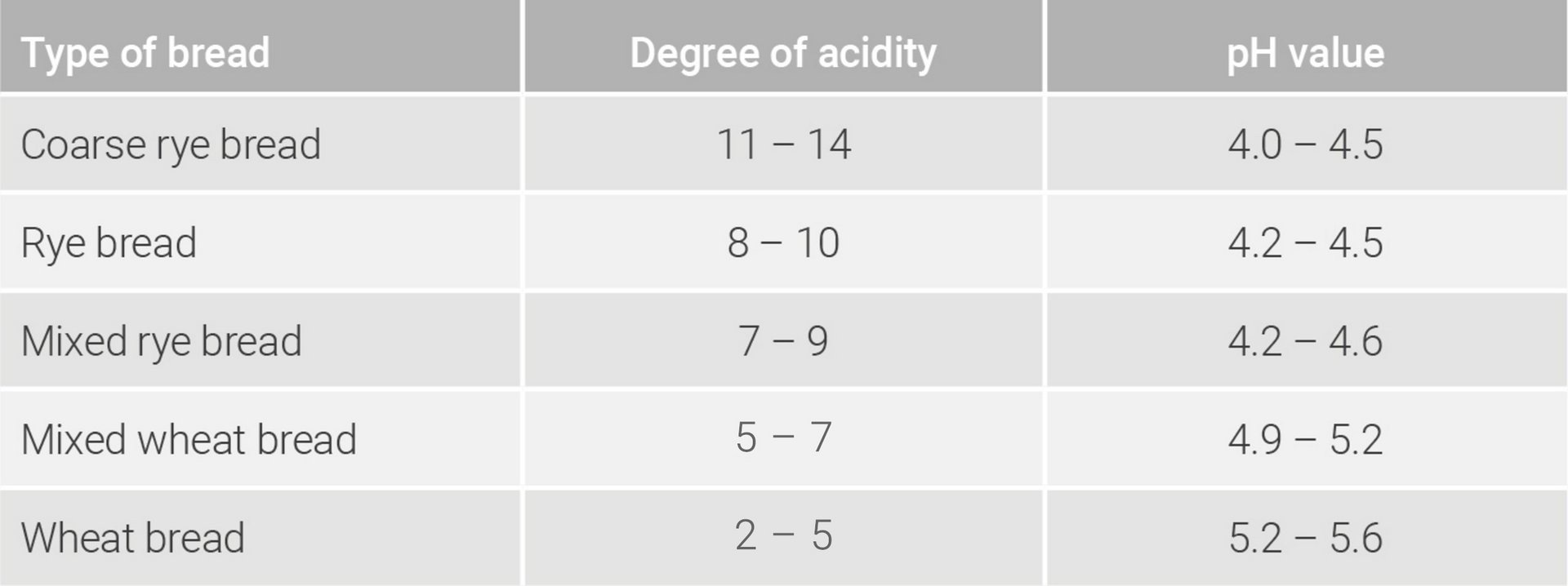
To determine the pH value, 10 g of a sourdough or a bread crumb with 5 ml acetone and approx. 40 ml of a total of 100 ml of distilled water are mixed in a mortar into a homogeneous mash. The mash is then transferred to a beaker together with the remaining 60 ml. Using a measuring electrode of a pH value measuring device, the pH value in the aqueous solution can now be determined. Should, in addition, the degree of acidity be measured, the aqueous solution is titrated so long with a sodium hydroxide solution (0.1 mol/l) until a pH value of 8.5 is obtained. The amount of sodium hydroxide solution (0.1 mol/l) used in ml/10 g sample, which is necessary to neutralize the acid in the aqueous solution, corresponds to the degree of acidity (Brand & Gänzle 2006; Meißner 2016). In the tables 8.1, 8.2 und 8.3, orientation values for pH values and degrees of acidity of various sourdoughs, bread doughs and types of bread are shown.